By Gary Victor, Sr. Manager, R&D at Viant
This article originally appeared in Medical Design and Outsourcing.
With health providers increasingly demanding single-use devices, the team successfully created disposable bone-cutting tools for its orthopedic device customers.
Within healthcare today, there is a heightened focus on “value” – on the episode of care and the need to deliver improved patient care outcomes and lower overall costs. A related trend in the orthopedic segment involves the role instruments can play and how they can ensure optimal clinical outcomes while mitigating expenses associated with post-operative infections.
Hundreds of thousands of hospital acquired infections (HAIs) occur in U.S. hospitals annually, requiring treatment costing tens of billions of dollars, according to U.S. Centers for Disease Control & Prevention. This, coupled with a growing requirement for instrument traceability, has led the orthopedic device industry toward disposable devices. These single-use solutions must live up to the promises of their original reusable versions to gain market acceptance. They must deliver on ever-critical performance, durability and value vectors.
As a leading medical device outsource manufacturing company, we have an important role to play. We’ve been stepping beyond traditional roles and proactively presenting unique solutions to our partners. Ultimately, we bring these products and technologies to market by engaging with programs through all phases of the development and production life cycle.
Our goal is to work through our customers to bring viable solutions to the medical device industry that enhance patients’ lives.
To help our orthopedic device company customers, we identified three clinical focus areas for organic product development: product traceability, consistent cutting tool sharpness and repetitive cleanliness. Based on the design team’s expertise in design control, unique manufacturing methods, material science knowledge and the state of the art mechanical testing labs, the obvious entry point was disposable bone-cutting tools. These high-use instruments are difficult to clean, require consistent sharpness and experience supply chain challenges in some markets such as rural settings and ambulatory surgery centers (ASC). The trauma market was also a natural fit due in part to the inability to forecast emergencies, which exacerbates supply chain issues.
Our engineers carefully evaluated our customers’ portfolios to identify gaps and launched research and development programs to meet those needs. We now have several programs underway in various phases of the development cycle and recently collaborated on a new product introduction by one of our partners. Products in development include a single-procedure intramedullary reamer, single-procedure patella reamer, single-procedure acetabular reamer and more.
Here are three important ways we’ve helped create single-use bone-cutting tools that perform as well as the reusable kind:
- Innovative design
Utilizing innovative design techniques, we changed the paradigm of cutting tools production without compromising performance. Each step of our approach required ingenuity. First we compared the performance of existing, reusable cutting heads to cutting heads of the same design manufactured with direct metal laser sintering (DMLS). Through extensive testing and analysis, we identified specific improvements that could be anticipated through the use of a metal injection molding (MIM) manufacturing process. We used rapid prototyping in multiple design-build-test iterations to optimize cutting performance. Using DMLS to optimize performance, we began using MIM to produce parts. Through creative mold tooling design, we produced low-cost, single-use cutting heads that require no additional post-manufacturing processes and have proven to perform as well as re-usable machined metal systems currently on the market yet at a lower cost.
- Using lower-cost materials without compromising performance
The durability of a cost-effective shaft represented the next challenge for the design team. Using predicate wire-wound and nitinol reusable shafts to set performance requirements, the team tested several lower-price options against established standards used by the reusable systems already on the market – and selected a carbon fiber cannulated shaft. To mitigate torque related performance when using the carbon fiber shaft intraoperatively, the team included two additional features. The first was a proximal torque slip mechanism that would control and limit amounts of torque that could be applied to the shaft. The second feature was an outer sleeve to provide a material barrier and fully encapsulate the shaft. These features ensure the product can be used safely and withstand aggressive use –without causing harm to the patient.
- Testing systems to prove we got it right
Equally important in designing and manufacturing our products is testing. When pursuing new technologies, we feel it is critical to characterize and compare performance and safety against currently used products. We used our deep knowledge and experience in material sciences and orthopedic cutting to design and produce custom test equipment and fixtures. Our rigor and focus around mechanical testing included the development of a truly unique powered delivery system that utilizes analytical and computer based simulations. These systems were leveraged to not only analyze current design performance, but also to help predict and evaluate alternative design performance. This allows us to demonstrate with absolute certainty that our single-use instruments are substantially equivalent to predicate re-usable devices, thus ensuring performance and patient safety is never compromised. (See the two charts with this article for examples.)
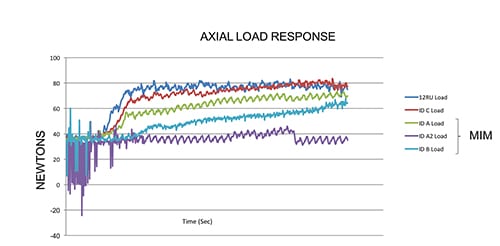
This chart shows how a predicate reusable device requires more force to cut the
samples than our disposable cutting head. [Image courtesy of Viant]
The result
Stepping out of a traditional outsourcer role to get closer to our customers and better understand their needs and constraints has enabled us to proactively provide innovative solutions that address some of the most pressing challenges in the orthopedic surgery market today.
In this case, we took an existing reusable product and re-engineered it for disposable use, which led to a new fully designed system. The comprehensive solution has received high praise from our market leading customer base and proven successful in numerous settings, including our and customer testing labs as well as co-sponsored cadaver labs.
While it is difficult to predict exactly where the market is trending in the coming five to 10 years, we are proving that by finding gaps in the instrument space, while also remaining aware of current market dynamics, it is possible to identify customer-enabling solutions.
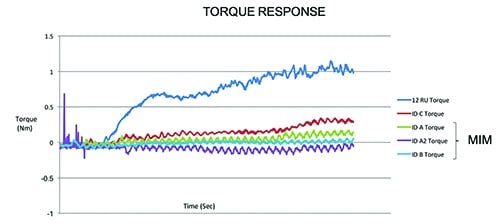
This chart shows how a predicate reusable device requires more torque to advance
when cutting samples than our disposable cutting head. [Image courtesy of Viant]
Gary Victor is senior manager of orthopedics R&D at Viant. Victor has expertise in
injection molding processing and product design, tool design, injection molding primary and
peripheral equipment, factory design and layout, equipment and tooling procurement, and
program management. He has bachelor’s and master’s degrees in mechanical engineering
from the University of Buffalo.
This article originally appeared in Medical Design and Outsourcing.