For Breakthrough Medical Devices, you need to…
Accelerate Product
Development
Commercialize & Scale
With Confidence
Optimize Design
for Manufacturing
Cut Costs without
Compromising Quality
Confidence in quality. Trust in expertise.
Whether you’re a major medical device OEM or a rapidly-growing startup, bringing a new device to market is full of challenges—tight timelines, regulatory hurdles, and the need to scale fast without compromising quality. Viant helps you stay ahead. We bring the engineering expertise and global manufacturing infrastructure to de-risk your product launch, optimize design, and scale seamlessly. Whether you need targeted support or an end-to-end solution, Viant is your agile, single-vendor partner to turn ideas into successful, life-changing medical devices.
Viant Delivers
Agile ingenuity to advance innovation
From global OEMs to early-stage innovators, Viant offers a unique a la carte approach—plugging into your development lifecycle anywhere or everywhere. Our global manufacturing seamlessly supports design transfer to full-scale production, filling resource gaps, solving problems and enhancing both clinical value and manufacturing efficiency to differentiate your device and drive success.
Proven programs to drive speed to market
Single-vendor reliability
End-to-end manufacturing capabilities
VERTICALLY INTEGRATED MANUFACTURING DRIVES EFFICIENCY & COST SAVINGS

Flexible partnership
Engineering excellence—exactly where you need it
Viant can deliver end-to-end services, but we don’t force broader engagements. We provide a unique a la carte approach—plugging in anywhere (or everywhere) in your development lifecycle. This allows us to fill resource gaps, problem-solve to break through barriers, and find smart ways to add both clinical value and manufacturing efficiency to your design to differentiate your device and drive commercial success.
Robust & proactive engineering support
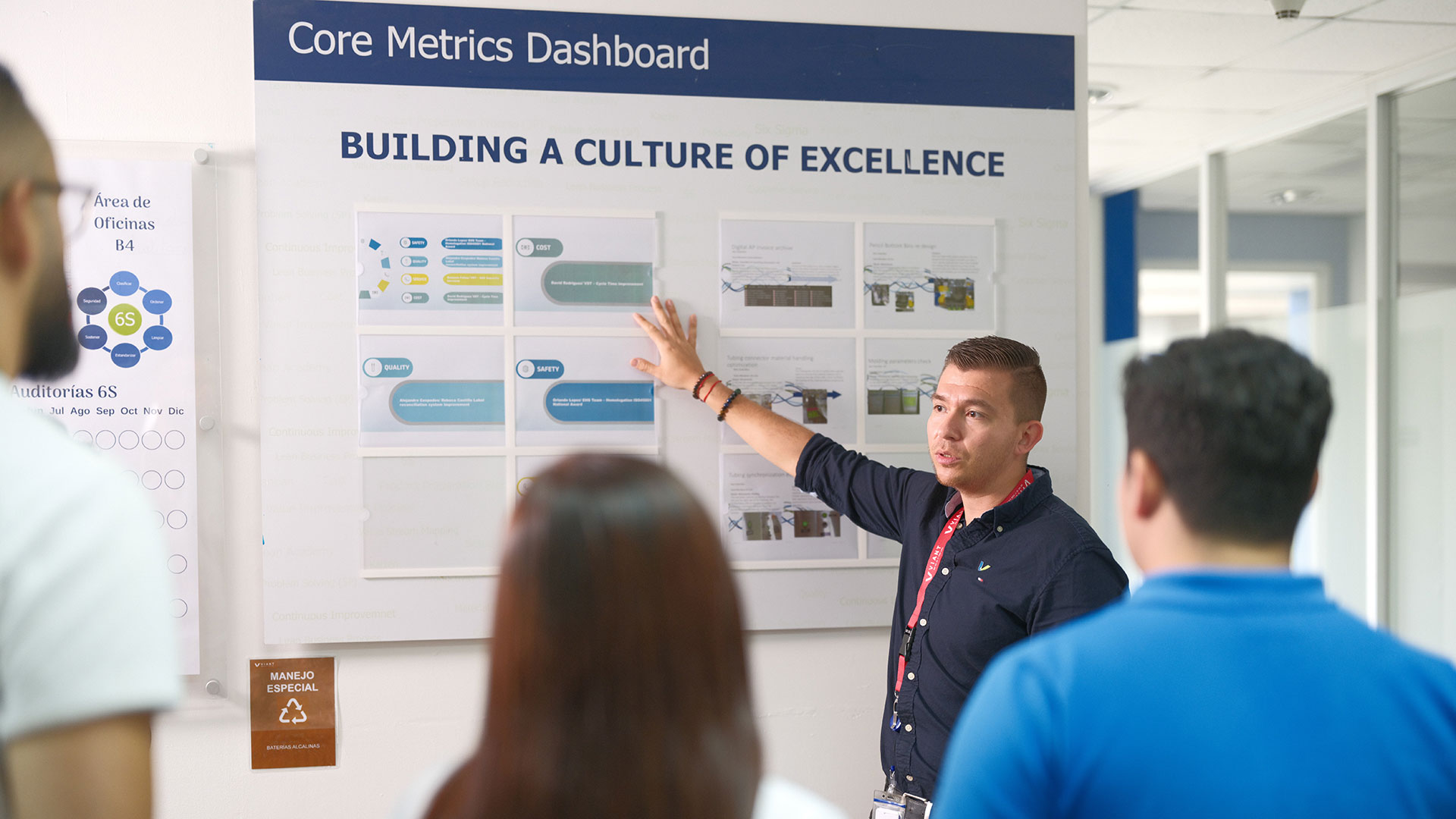
Accessible engineering expertise accelerates the product development lifecycle
Viant engineers aren’t reactive order-takers. We’re ready to engage early in the product development lifecycle. Our world-class D&D organization consists of proven innovators ready to incubate ideas and accelerate your path to product launch. And our Design-for-Manufacturing (DFM) experts directly integrate with your product team to optimize product design for clinical functionality, production reliability at scale and cost.

100% focused on med tech
Bringing INNOVATION to life ACROSS CLINICAL Markets
At Viant, our sole focus is medical technology—but our expertise spans clinical applications and specialties. Our deep understanding of clinical markets enables us to build solutions that navigate unique demands and challenges of your field. This allows us to deliver devices that maintain the highest quality standards, while improving patient and provider outcomes.
Orthopedics
We are at the forefront of orthopedics, providing complete solutions including instruments, implants and delivery systems. Our orthoplastics group is a global leader in UHMWPE components.
Surgical Technology
We help with a range of clinical markets and applications when it comes to Surgical Technologies, including minimally invasive surgery/robotics, endoscopy, ophthalmology, wound care, biopsy, aesthetic and general surgery.
Cardiac & Interventional
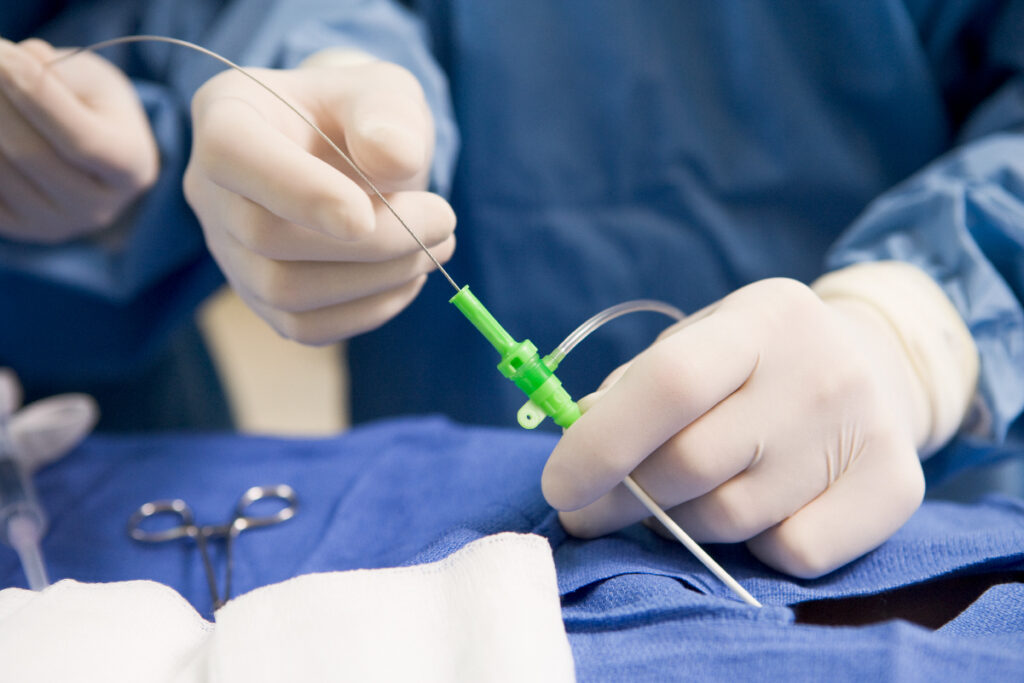
Highly skilled in the design, development, and complex assembly of a wide range of cardiac and interventional devices. Our specialized capabilities include: overmolding and nitinol tubing. We leverage both tubing and surgical technology expertise to manufacture precision hypotube components for cardiac and interventional applications.
Drug Delivery
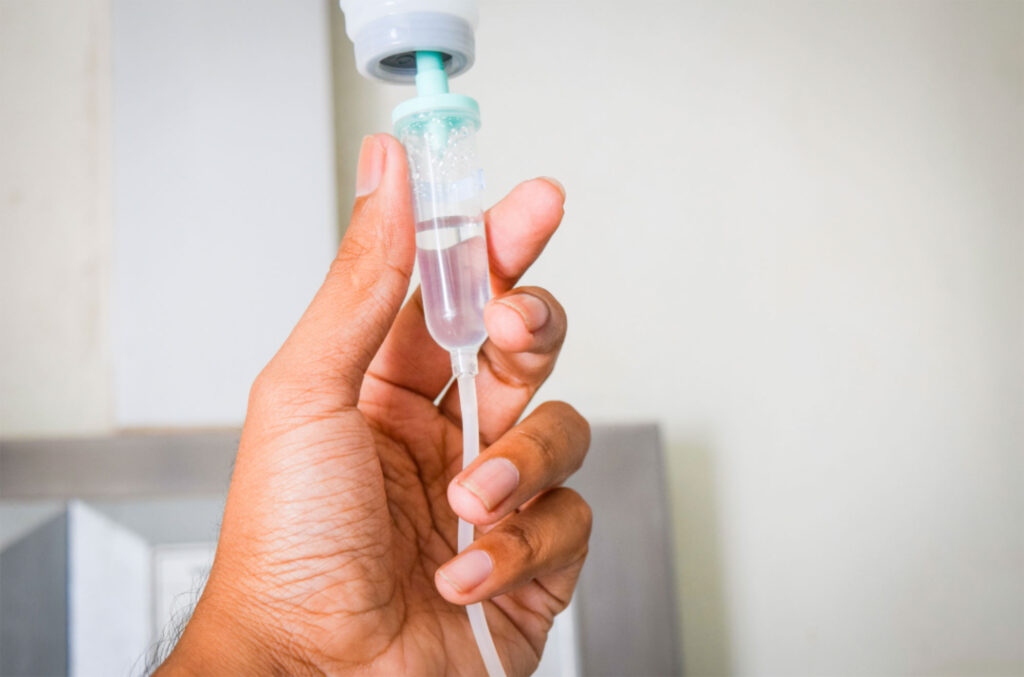
Our team supports a range of technologies, including wearable and non-wearable auto-injectors, IV sets and connectors, respiratory devices, and more. Our end-to-end solutions give us full control over every step of the process, such as filling, packaging and sterilization management.
Bioprocessing
Bioelectronics

We cover a wide swath of clinical markets and applications when it comes to Bioelectronics, including pain management, sleep apnea, OAB/incontinence, cardiac therapeutic/diagnostics, immunological conditions and wearables.
Diagnostics & Laboratory Products

Respiratory, Monitoring & Patient Care
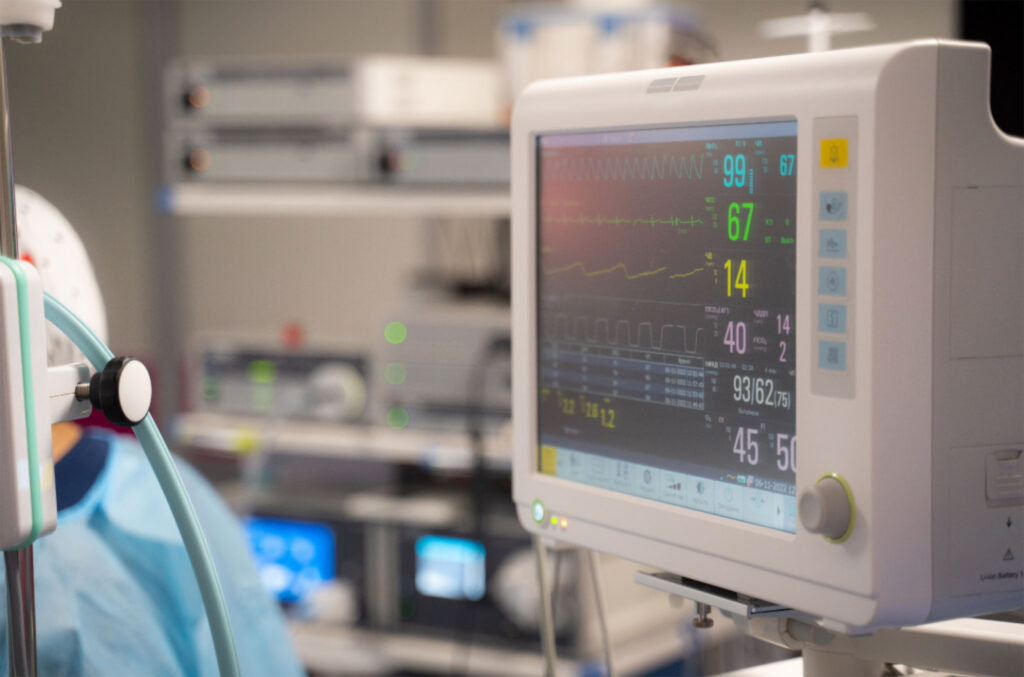
We have a wide range of capabilities including both plastic and silicone molding. Our capabilities include manufacturing and assembly of masks, trach tubes, and airway tubes, as well as console components and sub-assemblies.
globally
square feet of
factory space
square feet of
clean room space
focused on delivering
for our customers
Supply chain excellence
Intelligent supply chain & capacity planning to avoid disruptions
Proven scale & capacity
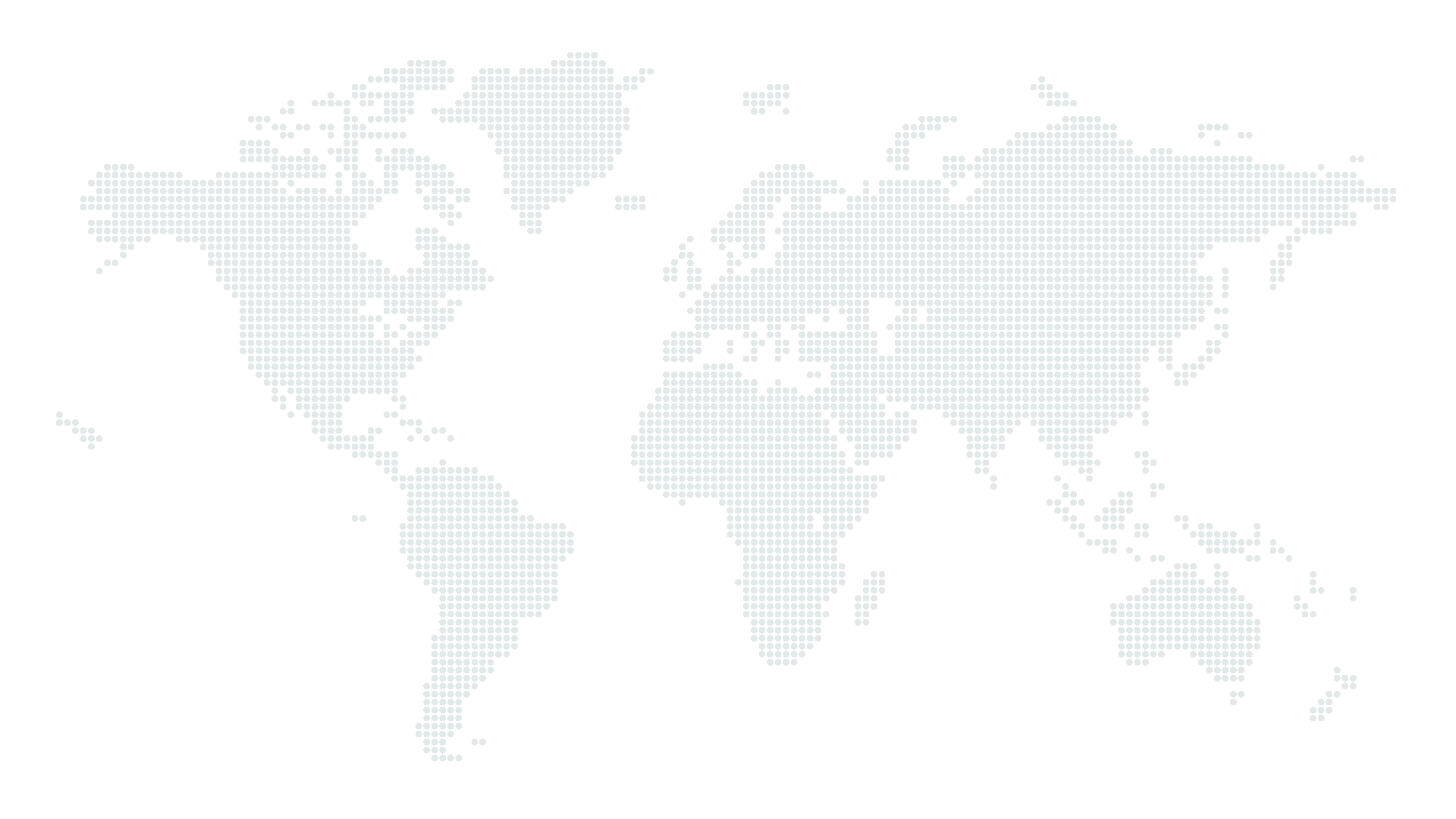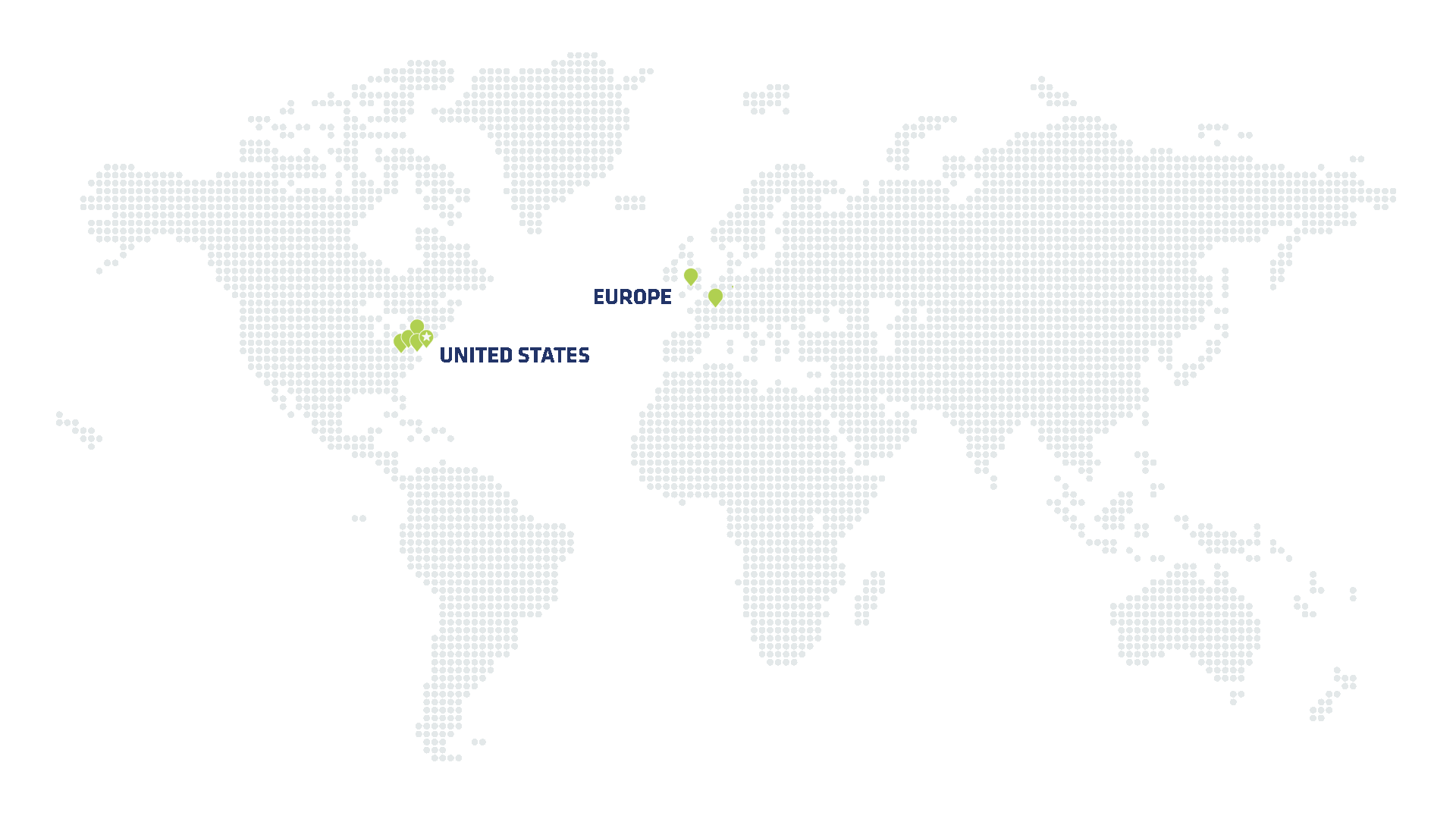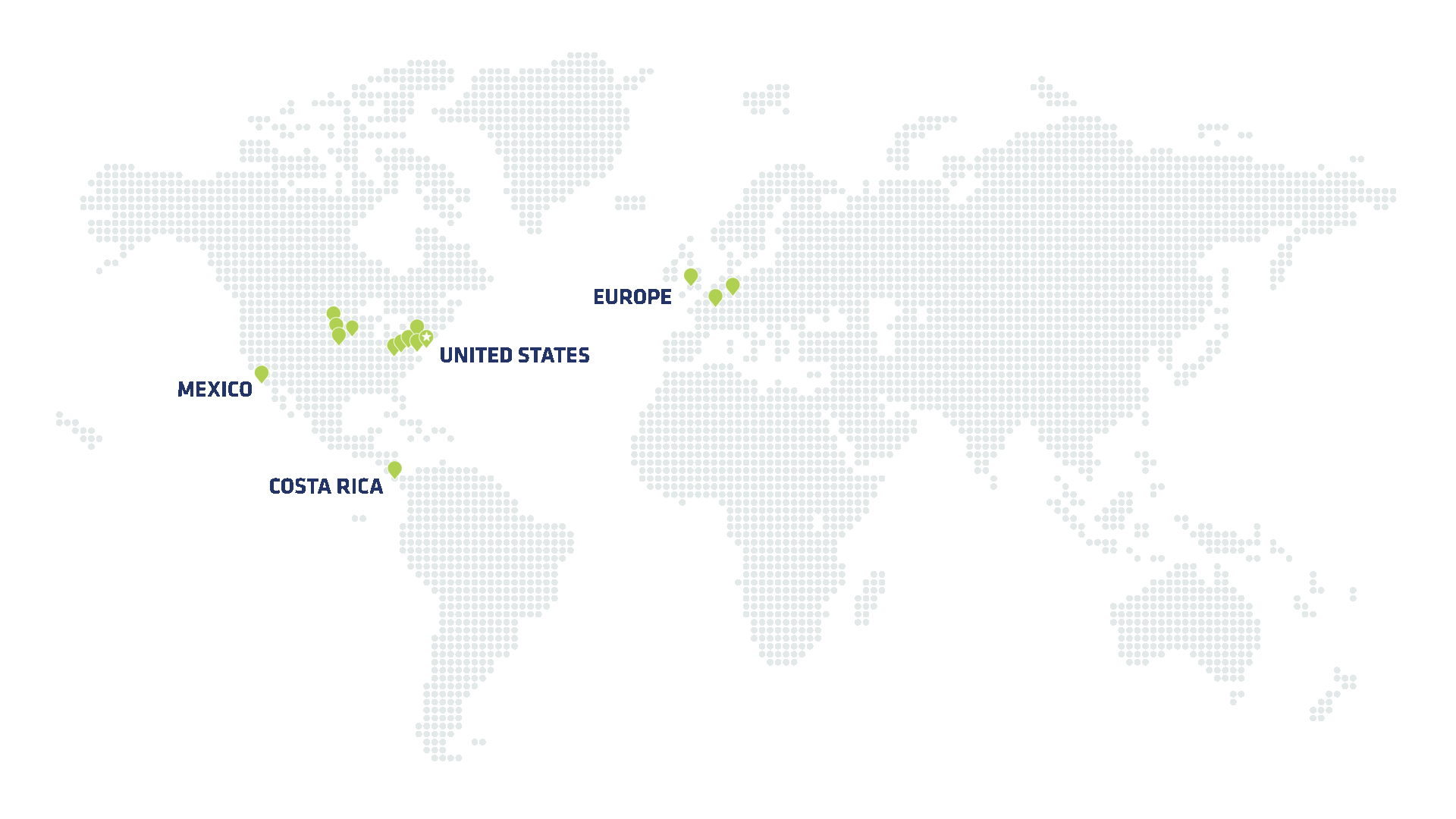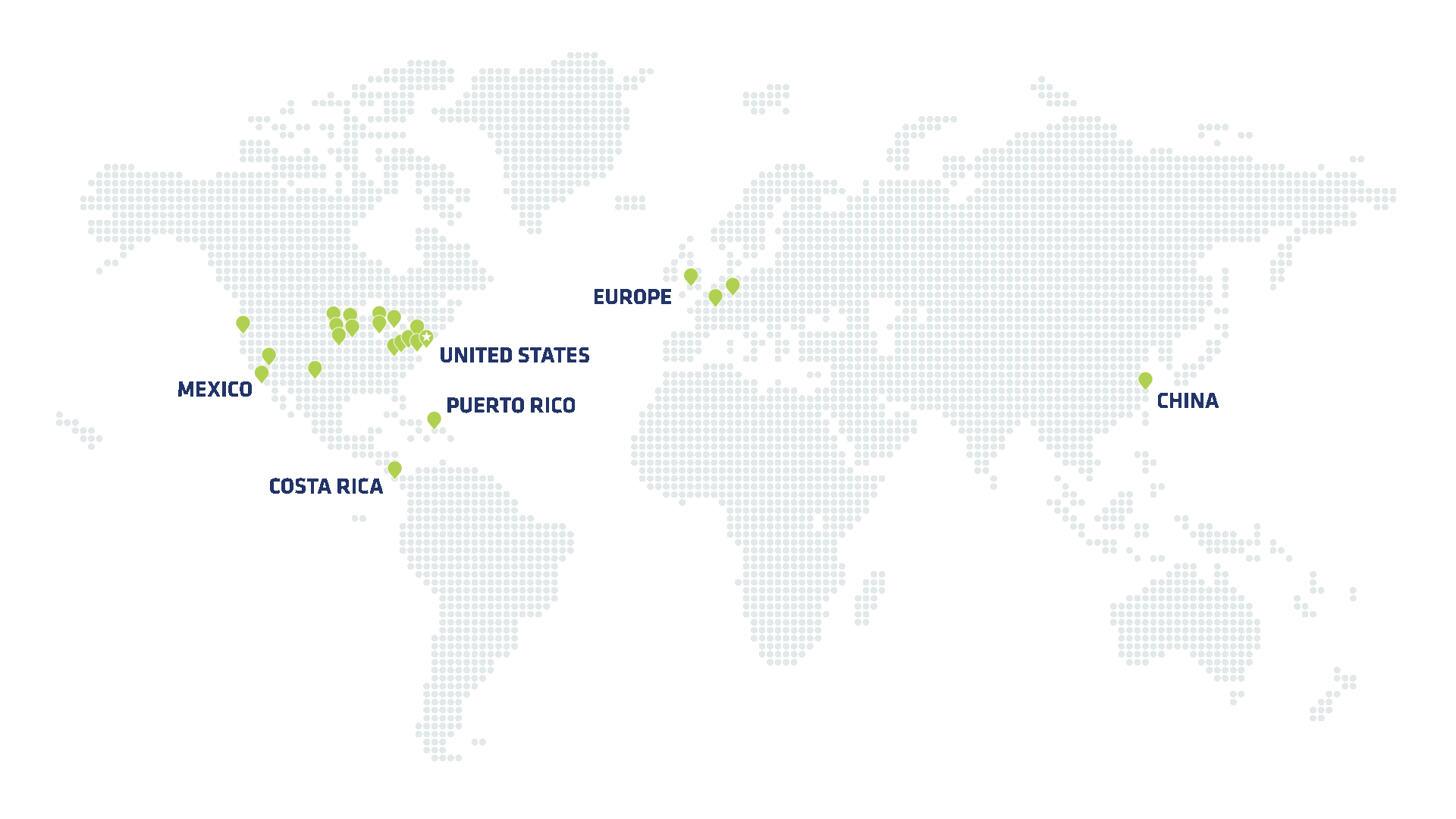
Global footprint for responsive scale at speed
Speed to market is critical for initial product launch—but also for scaling up production to meet growing demand. Viant has spent decades building out a global manufacturing network. That scale gives our customers the flexibility to onshore production, leverage regional production where necessary and access our full global footprint for smarter supply chain and distribution. Just as importantly, our robust manufacturing infrastructure means Viant is ready to scale at speed—while maintaining speed at scale.