By Mary Carmen Luna Matuk, Head of LATAM, Pharmaboardroom
“In-conversation: Migna Fontan – Director of Operations, Viant Puerto Rico” is an Article from Pharmaboardroom, and will appear in Puerto Rico Healthcare and Life Sciences Review 2025.
Migna Fontan, Director of Operations for Viant Medical in Puerto Rico, provides insights into the company’s evolution and its critical role in the MedTech industry. Fontan discusses Viant’s journey in Puerto Rico, its transformative milestones, and its manufacturing facility’s innovative capabilities. She highlights how the island’s skilled workforce positions Puerto Rico as a vital hub for the global MedTech sector.
Could you begin by sharing an overview of Viant and its journey in Puerto Rico?
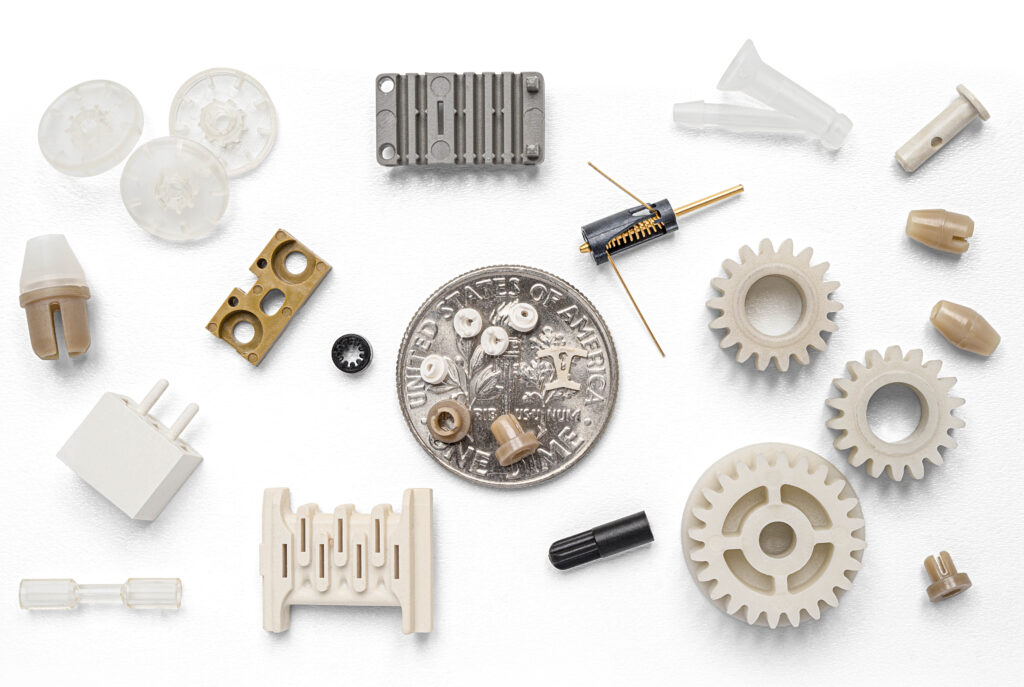
Viant Medical is a leading medical design and manufacturing partner, trusted by OEMs to increase speed to market while elevating product quality and performance. We operate globally with 25 locations worldwide to provide service in design and development, material expertise, medical device components, manufacturing and assembly and finished device services, including packaging, sterilization management and logistics management.
Our facility in Puerto Rico has been in operation since 1990. Initially focused on Injection Molding, our site has continued to expand its expertise and capabilities over the years. Alongside well-established proficiencies in over molding and two shot molding, we are proud to announce the integration of combination drug delivery device manufacturing, showcasing our commitment to innovation and excellence.
I am very excited to be part of the site transformation and look forward to continuing to help Viant’s partners improve patients’ lives.
Viant has had a presence in Puerto Rico since 1990, and you have been with the company for a significant portion of that time. What major changes or milestones have you witnessed during these years that stand out as transformative or particularly noteworthy?
Viant’s operations have evolved significantly to meet the changing demands of the industry and our customers. One key milestone was the diversification of our facility capabilities. We initially focused solely on injection molding but have now expanded to include sub-assembly and assembly of finished medical devices. Additionally, the implementation of our capabilities to manufacture combination drug delivery devices has positioned us as a leader in this challenging area. The adoption of new, state of the art manufacturing and automation technology enhances our processes, providing tailored solutions and support with global scalability to our partners. We are continuously enhancing and strengthening our quality and compliance, reflecting Viant’s commitment to our partners and the patients they serve. Another important focus has been increased investment in talent development; investing in our resources and integrating a culture with a clear mission, vision and values have been key drivers for our success.
Could you outline the unique features and capabilities of Viant’s manufacturing facility in Puerto Rico?
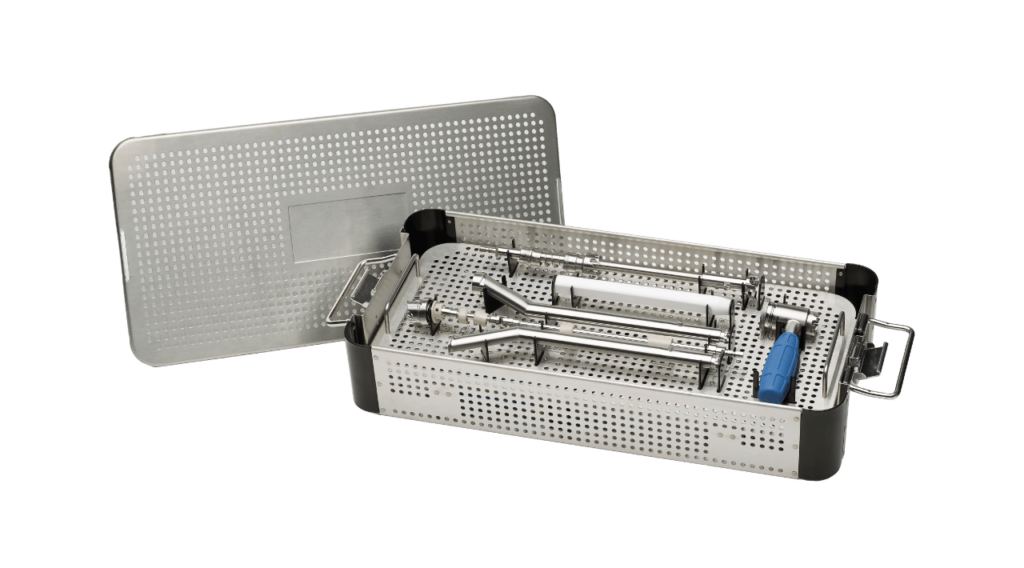
Viant’s manufacturing operations stand out for our expertise in managing all aspects of the product life cycle: from design and development, to manufacturing, assembly, finished device services and delivery to the distribution center. This comprehensive approach enables us to streamline processes, ensure strict adherence to quality and compliance, and accelerate programs to market. All these capabilities are managed under one roof, offering our customers optimal efficiency, reduced complexity, and the agility to react and adapt to ever-changing markets and demand.
Have any notable innovations emerged from your operations here in Puerto Rico?
Viant’s commitment to innovation is exemplified by the successful collaboration between our engineering team, customers, and SMEs through a standard methodology called 3P – Production, Preparation, Process. This approach enables us to design and implement cutting-edge automation solutions tailored to the needs of each device program. We also have many examples where the integration of the advanced technologies at our facility has optimized efficiency, enhanced quality and streamlined production for our partners.
How does the Puerto Rico facility align with and contribute to Viant’s global strategy, given the company’s extensive network of manufacturing sites worldwide?
The Puerto Rico site plays a key role in our global strategy by leading the implementation of combination drug delivery manufacturing services, a new capability for Viant. This site was strategically selected among 25 facilities worldwide due to its proven track record of excellence, strong KPIs, advanced capabilities and highly skilled workforce. The Puerto Rico site is recognized as always driving innovation, maintaining regulatory compliance, and delivering high quality solutions to meet our customers’ requirements.
What would you like readers to understand about Puerto Rico’s value in the MedTech industry and its relevance to Viant’s operations?
Puerto Rico offers significant value to the MedTech industry. It is recognized for manufacturing medical devices with the highest standards of quality, with an emphasis on excellent customer service. Most importantly, we have a highly skilled workforce that can meet the diverse needs of the MedTech sector. These three elements—quality, service, and talent—are the foundation of what Puerto Rico offers to our customers.
There is a growing effort to position Puerto Rico as a competitive hub for contract manufacturing. We have excellent educational institutions, consistently producing skilled professionals who are ready to excel in this field. At Viant Puerto Rico, we have many success stories that demonstrate the effectiveness and potential of this region in supporting the MedTech industry.
Many of your industry colleagues highlight Puerto Rico’s highly skilled workforce as a significant advantage. How does Viant effectively retain talent in such a competitive environment?
Viant has a comprehensive human resources strategy focused on talent retention. We focus on development and work closely with our associates to create career development plans in collaboration with their supervisors. Through our emphasis on career ladders, which provide a clear path for advancement, we have supported numerous success stories of employees growing from entry-level roles to management positions. Recognition and performance-based rewards are also essential to our retention strategy. This includes site-level awards and corporate-level Value Awards, which highlight outstanding contributions across the entire organization, ensuring associates’ hard work and positive impact is recognized and celebrated.
By aligning associate development opportunities with Viant’s vision, providing clear career pathways, offering robust training, and fostering a culture of recognition, we’ve created an environment where talent feels supported, motivated, and empowered to thrive.
Finally, what message would you like to convey to our global readers about Viant and Puerto Rico’s role in the global MedTech industry?
At Viant Medical, we live our values in all we do. Our mantra is “We’re in it for Life”, and this truly reflects our goal to partner and innovate with our customers to provide the highest quality, life enhancing medical devices in the world. Viant’s mission is to be the medical device industry’s most trusted design and manufacturing services partner.
What sets Viant apart are our comprehensive, end-to-end capabilities. From design and development to manufacturing, assembly, and final product delivery, we manage every aspect of the product life cycle. This holistic approach allows team members to provide unmatched service and adherence to quality standards. Puerto Rico plays a vital role in supporting this mission, and we are proud to contribute to the global medtech industry from this dynamic hub.