$8 million investment, including new building & FDA-approved vacuum plasma spray (VPS) equipment, enhances in-house coating and single-source orthopedic implant capabilities
FOXBOROUGH, Mass., May 17, 2021 — Viant announced today that it has completed a more than $8 million expansion at its Orthopedic Implant and Coating Center of Excellence in Chaumont, France. The expansion includes a new building with a controlled-environment room and an investment in vacuum plasma spray (VPS) coating technology that recently received approval from the US Food and Drug Administration (FDA). The upgrades enhance Viant’s capabilities as a single-source supplier and gives it immediate capacity to partner with additional orthopedic customers.
Take a virtual tour of Viant’s Chaumont facility:
- Take a look at our Chaumont Facility tour.
Read a case study about Viant’s Chaumont Facility:
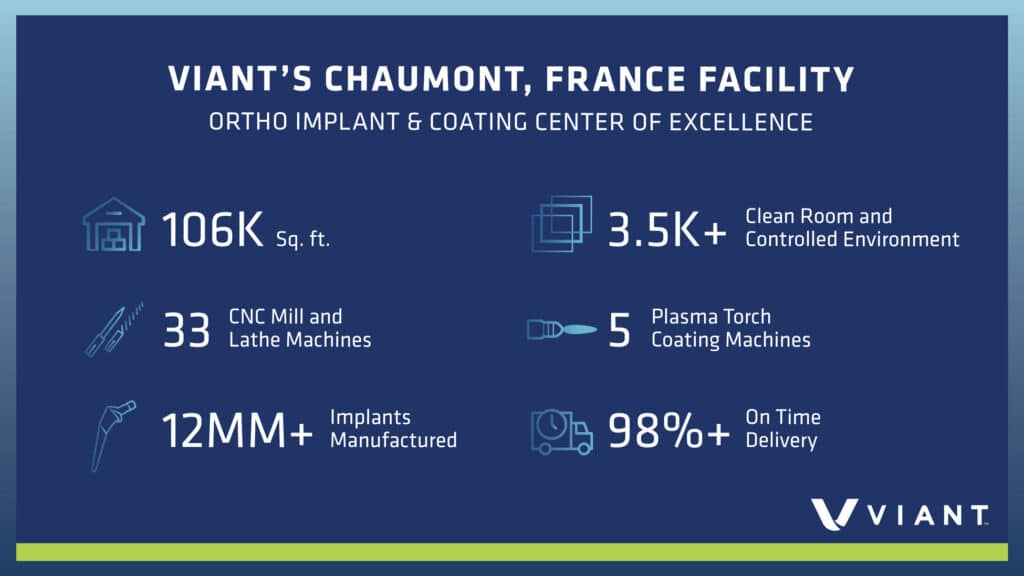
In-house VPS coating capability will enable the application of titanium coatings or a dual layer of titanium and hydroxyapatite (HA) coatings. Applied to orthopedic implants such as hips, knees and shoulders, these coatings add friction to help anchor the implant and provide a surface that promotes long-term bone growth and healing. VPS technology allows these coatings to be applied in a thickness that optimizes performance. This new VPS capability complements the Chaumont facility’s existing HA coating capabilities, with a track record of more than 8 million implants coated.
Viant’s investment includes a new 8,600-square-foot building and an ISO Class 9-equivalent, controlled-environment room with the new VPS torch and related equipment, as well as operations including grit blasting, shot peening and surface finish measurement and inspection.
Viant’s state-of-the-art, vertically integrated Chaumont facility has more than 30 years of experience transforming raw materials into products ready for the operating room. It features:
- Metal and plastic machining & fabrication
- Polishing
- Subassembly
- Cleaning
- Laser marking
- Coating
- Assembly
- Sterile packaging
- Microbiology laboratory
Focusing on customer service, the facility offers short lead times and maintains 98%+ on-time delivery rates.
About Viant
Viantis a global medical device design and manufacturing services provider that partners and innovates with customers to provide the highest quality, life enhancing medical devices. We do this through our depth and breadth of capabilities, end-to-end integration, technical expertise, and relentless focus on our customers and on operational excellence. With nearly 6,000 associates across 24 locations worldwide, we offer a unique combination of small-company service and attention with big-company resources. For more information, visit viantmedical.com or follow us on LinkedIn.