Viant: Company Overview

Precision Plastics for Complex Devices

Site Tour: Grand Rapids, Michigan

Site Tour: Tijuana, Mexico

Site Tour: Fremont Micro-molding

Site Tour: Indianapolis, Indiana

On a Silver Platter: Examining the Cases & Trays Market
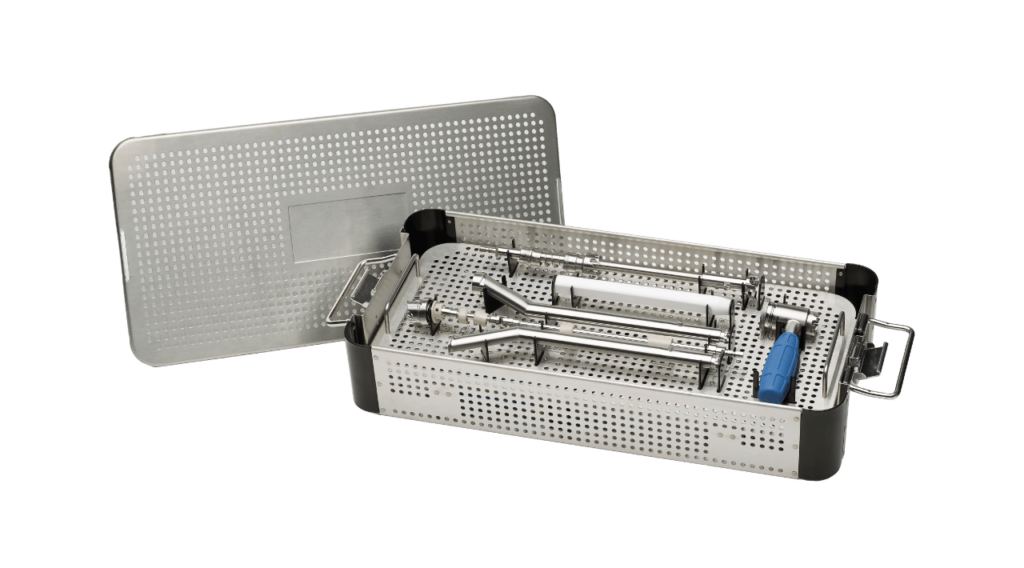
By Mark Crawford, Contributing Editor This article originally appeared in Orthopedic Design & Technology Growth is strong in the cases and trays (C&T) orthopedic market, especially in the extremities sector. For example, the overall global procedure trays market size was valued at $17.6 billion in 2022 and expected to expand at a compound annual growth […]
Vertical Integration Speeds Scale-up for US Launch of Orthopedic Implant
Single-source supply and end-to-end solutions guide major improvements to production capabilities and create efficiencies for a European medical device manufacturer.
Video Overview: Motion Preservation Spinal Implants

Site Tour: Grand Rapids, Michigan

Collaborative Transfer of Surgical Device Meets Aggressive Time Frame

A leading, multinational medical technology company needed to transfer manufacturing of a medical device to free up cleanroom manufacturing space for a new product.
5 Questions for Viant at Medica/CompaMed 2019

By Sean Fenske This article originally appeared in Medical Product Outsourcing Magazine. Once again, I find myself wandering the many halls of the Medica and CompaMed trade events. Between checking out the latest and greatest from the medtech elite, discovering new firms displaying their first products at the show, or catching up with those I’ve met […]
