
your trusted manufacturing services partner
Together, let’s save and extend lives.
Our network of dedicated local resources and enterprise expertise can help solve the most complex design and manufacturing challenges.

A message from our CEO, Alton Shader

Trust is everything for us. It’s the core of our vision and it comes through in everything we do.
When you work with Viant, our team is your team. Quality manufacturing, accountability from the bottom-up and top-down, and a trusted partnership you can count on from day one.”
25
Locations Worldwide
2.3M
Square Feet of Facility Space
500+
Precision Machining Centers
300K+
Square Feet of Clean Room Space
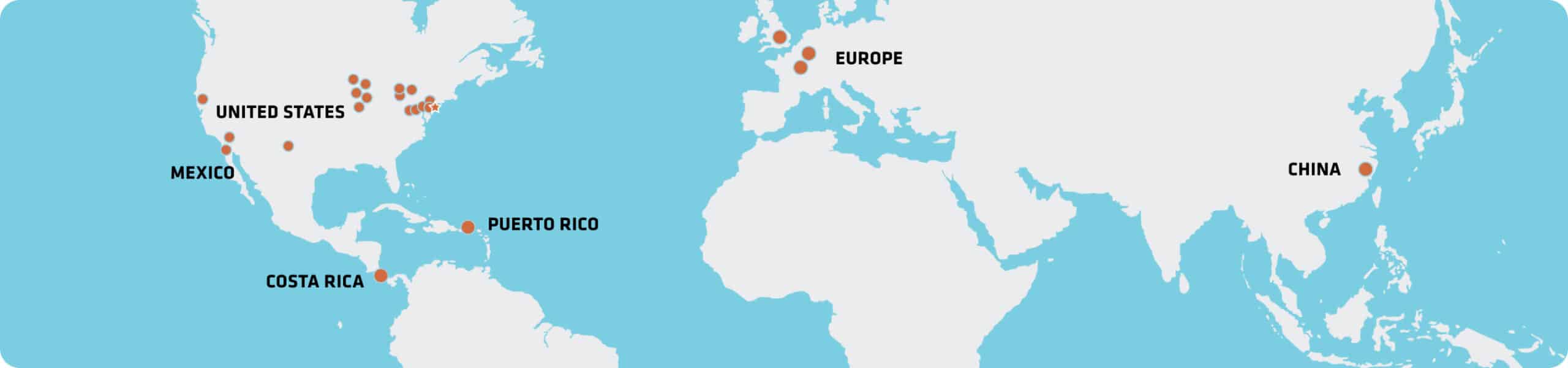
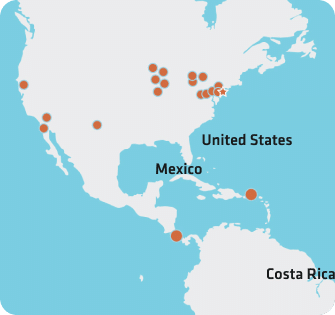
Our Locations
Quality at a Global Scale
Strategically located near key medtech hubs, our facilities blend enterprise resources with personalized attention to help our customers meet their goals. Whether it’s nearshore manufacturing, local-for-local, or specialized expertise, our global network can support your needs.
About Viant
Our Story
Today’s Viant has roots that go back more than five decades. Since our first facility entered the medical device market in 1968, we’ve been strategically expanding our capabilities to offer our customers a complete, end-to-end solution for their medtech needs.
1968
Viant enters the medical device market with precision metal tubing.
1970
Viant enters the orthopedic market.

1979
Viant opens its first molding facility.

1985
Viant begins assembly of complex medical devices with an initial focus in surgical devices.
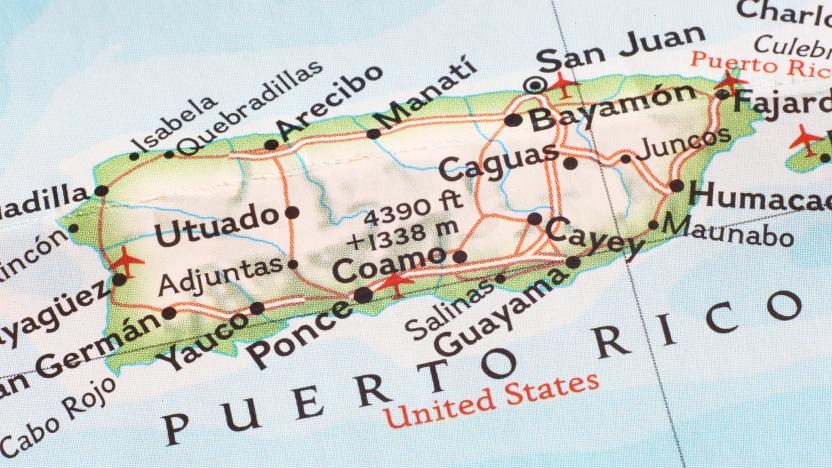
1990
Puerto Rico facility opens, growing molding & assembly capabilities and offering a low-cost manufacturing option.

2000
Viant offers Design & Development as a capability for the first time.
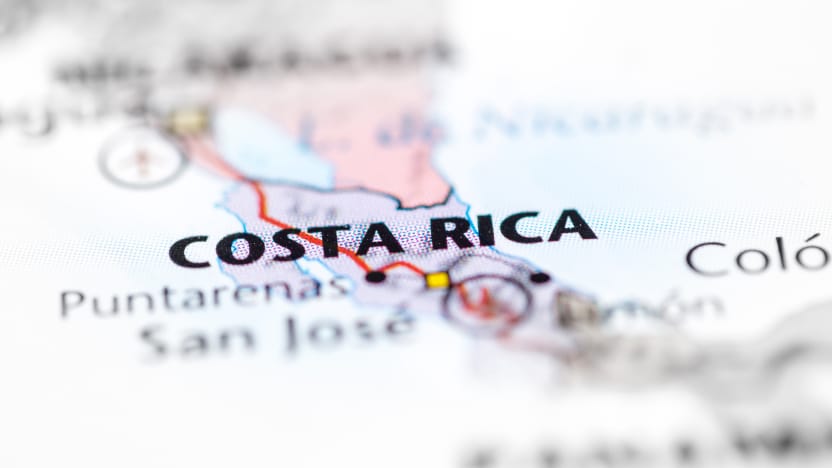
2004
Low-cost country manufacturing capabilities expand through the addition of our Costa Rica facility.

2005
Viant offers specialized UHMWPE solutions for orthopedic implants via their Orthoplastics division.

2012
Viant expands precision plastic products expertise with the acquisition of facilities in the U.S., China, and Mexico.
2017
Viant greatly expands footprint and capabilities by more than doubling in size through acquisitions.

2018
Company changes its name to Viant to better reflect expanded range of end-to-end solutions for the medical technology industry.

2018
Viant expands its holdings in advanced surgical and orthopedic product lines.
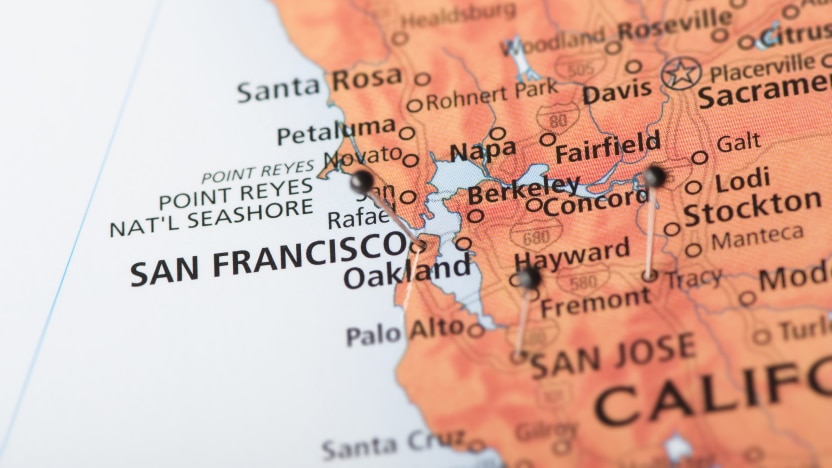
2019
Viant acquires West Coast D&D hub and expands interventional & bioelectronics expertise.

2021
Viant adds the specialized capabilities and environmental controls to manufacture combination products within the drug delivery market at its Puerto Rico facility.

2021
Viant enhances in-house coating capabilities with major expansion of Chaumont manufacturing facility.

2022
Viant supports new growth with second major expansion of Costa Rica manufacturing facility in two years.

2022
Viant expands its West Coast D&D hub, co-locating with its manufacturing facility to create a San Francisco Bay Area campus and Complex Extrusion Center of Excellence.
READY TO PARTNER?
Have a tough question? We’re ready. Challenge us with your complex problems, today.
